Precision Microbiota Engineering for Child Health
Two very serious diseases of newborns – namely, inborn errors of metabolism and neonatal sepsis – currently have high mortality rates, long-term consequences for child development and limited treatment options. The consortium seeks to develop novel intervention strategies for these diseases using advancements in microbiota bioengineering.
From shortly after birth, the large intestine is colonised by billions of bacteria, which make up the intestinal microbiota. Researchers have only recently begun to understand the extent and the mechanisms by which these bacteria influence child health and development. However, current studies support the causal roles of bacteria in diseases as diverse as allergy and autism. Despite this knowledge, we still have no accurate medical intervention to “fix” the microbiota.
The focus of this project is to develop novel tools to precisely engineer the microbiota of individuals with inborn errors of metabolism or neonatal sepsis and to develop essential tools to monitor the efficacy of these interventions. The project aims to alter the metabolism of the microbiota or to replace “bad” bacteria or functions in the microbiome of the gut with “desirable” ones. This modification will be achieved with diverse methods that range from the latest state-of-the-art molecular biology and antibody engineering technologies to low-cost oral vaccines. This will be combined with a detailed analysis of tissue scarring, single-cell analysis of organ development, a novel medical imaging approach and quantitative analysis of brain function, in order to assess the therapeutic efficacy of the interventions on long-term development and health. This range of expertise allows not only to develop microbiota engineering technologies, but also to develop detailed mechanistic insight into how the technology improves health at a molecular and cellular level. We hope that these methods will allow antibiotic-independent selection of successfully modified bacteria, generating a robust and predictable change in microbiota function/composition.
Overall, fundamental insight into microbiota, and preclinical insight into therapy efficacy and safety will be generated. Our major tools can be produced, distributed and administered in low-cost and low-technology environments, smoothing the way to implementation. Moreover, since microbiota engineering can be applied across a wide range of childhood diseases, this effort has far-reaching implications for the future of medicine.
Above banner image: Microscopic view of a healthy mouse colon. F-Aktin, shown in red, highlights the smooth muscle layer as well as the luminal border of the epithelium. Cell nuclei are shown in blue, with a small percentage of nuclei being co-stained with a proliferative marker. The number of co-stained cells helps researchers to draw conclusions about the inflammation severity of the tissue.

Two researchers examine culture results on petri dishes.
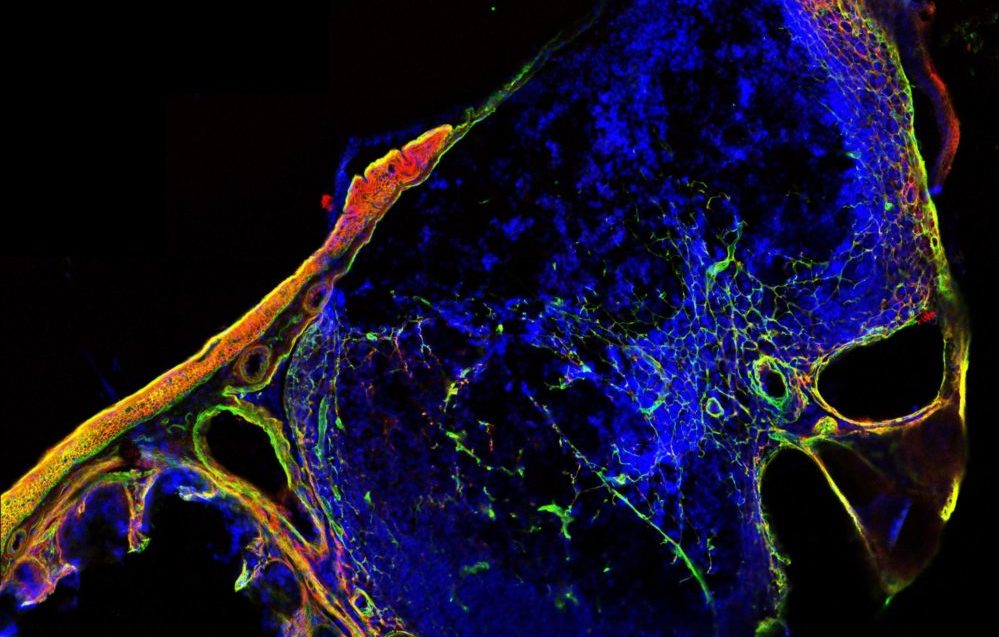
Microscopic view of a cross-section through a lymphoid structure in the mouse cecum, showing extracellular matrix in green and red and cellular nuclei in blue. Image: Ronja Rappold

A researcher looks through the lens of a powerful microscope. Novel imaging methods allow the consortia to study the microbiome in the tissues of the gut.
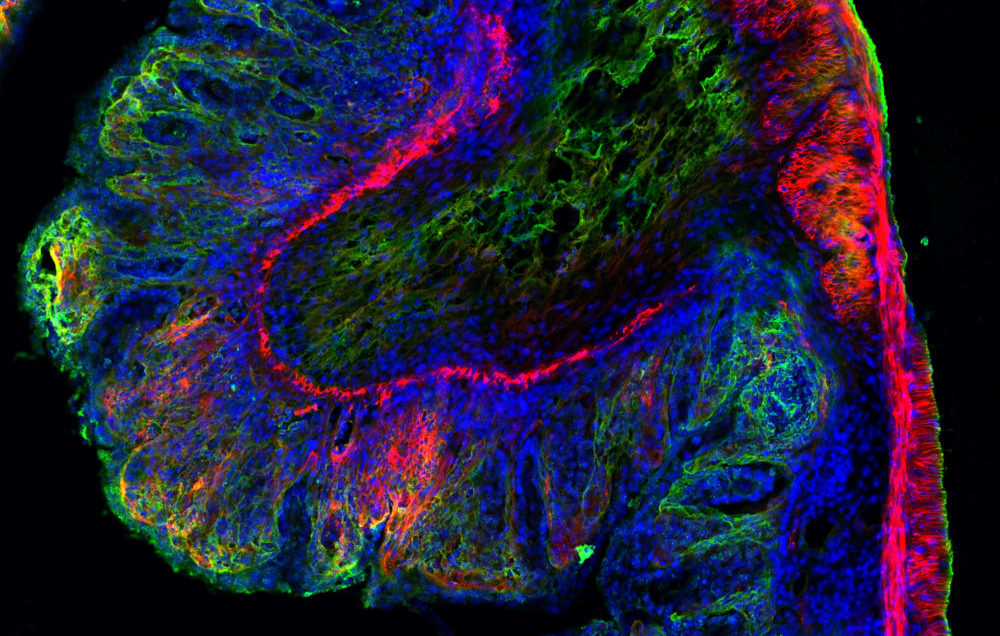
Microscopic view of Salmonella typhimurium infected mouse digestive tract tissue showing extracellular matrix in green and red and cellular nuclei in blue. Researchers study these types of images to understand how harmful bacteria affect the gastrointestinal tract. Image: Ronja Rappold

A researcher prepares samples in an oxygen-free tent for in vitro investigations of human gut bacteria, the gastrointestinal microbiome.
Project Overview: Prof Emma Slack speaks about the research project and its goals.
Additional Information
- The research is part of the BRCCH Multi-Investigator Programme.
- The consortium is led by investigator Prof Emma Wetter Slack.
- Additional investigators and collaborators include Prof Matthias Baumgartner, Dr Martin Behe, Prof Johannes Bohacek, Prof Médéric Diard, Prof Adrian Egli, Dr Sean Froese, Prof Johannes Häberle, Prof Giancarlo Natalucci, Prof Shinichi Sunagawa, Dr Johannes Trück, Prof Viola Vogel, Prof Ferdinand von Meyenn and Prof Christian Wolfrum. Consortium members include Mario Benn, Elisa Cappio Barazzone, Charlotte Fonta, Dr Noëmi Rebecca Meier, Ronja Rappold, Carine Roese Mores and Rudolf von Wartburg.

