Science Speaks: Conversations on Health Podcast - What is Pathology?

There are numerous examples of pathologists in books, films and television shows, but how accurate are these portrayals? In the first episode of Science Speaks: Conversations on Health, Vicky Edkins from the Basel Research Centre for Child Health talks to Professor Alexandar Tzankov, a surgical pathologist at the University Hospital Basel. Learn more about the fascinating work of pathologists – from unravelling the impact of COVID-19 on the body to uncovering the cause of death of a mummified lady found in a Swiss church.
Listen on Spotify, Apple Podcasts or YouTube.
Download the full episode transcript here or read below.
What is Pathology? with Professor Alexandar Tzankov
[Vicky Edkins]
Welcome to the Science Speaks: Conversations on Health podcast. This podcast series has been developed by the Basel Research Centre for Child Health in Switzerland to share some of the great work that is taking place and look at what it means to be a scientist within the various different disciplines that are represented by the Centre.
In this episode, we are delighted to be talking to Professor Alexandar Tzankov at the University Hospital Basel. Professor Tzankov is a surgical pathologist as well as the Head of the Department of Histopathology and Autopsy at the Institute of Medical Genetics and Pathology here at the University Hospital. Professor Tzankov, thank you very much for joining us.
[Prof Alexandar Tzankov]
Thank you very much for this kind invitation, I highly appreciate it.
[Vicky Edkins]
I would like to start this podcast with a discussion of what your job involves. We asked a few members of the public what they thought pathology is, or what they thought the job of a pathologist involves. We've assembled so me of the responses that we got to these questions and we're going to play them to you now. And then perhaps it'd be really great if you can reflect on how well they match the reality of your job.
[Speaker 3]
I think a pathologist is a type of doctor who examines bodies and who studies specific types of diseases and illnesses.
[Speaker 4]
I think a pathologist performs autopsies and supports police and detectives in solving crimes.
[Speaker 5]
I think a pathologist works with people to look into different conditions of the human brain and body.
[Speaker 6]
A pathologist is a scientist who studies different diseases, types of diseases and typologies of diseases.
[Speaker 7]
Pathology, isn't that someone who looks at dead bodies to figure out how they died?
[Vicky Edkins]
So Professor Tzankov, what's your impression of these statements? Do you think they reflect accurately what your job involves?
[Prof Alexandar Tzankov]
They to a part reflect what I'm doing, but they also reflect some perceptions gained from the TV or from other mass media. So at least in Europe, in continental Europe, pathologists do not really support the police. This is the job of legal medical doctors, which is quite a different specialty in clinical medicine.
But the answers of the people quite nicely reflect how pathology developed out of a medical specialty dealing with causes of death, examining dead bodies from the perspective of the macroscopy of visible organ changes, which then underwent kind of an evolution, looking at the microscopic appearance of diseases causing death and then realizing that those microscopical appearances of diseases can be used even for living people to precisely assign diseases, whether being inflammatory, infectious or tumorous nature, and then detecting special changes at the microscopical level, enabling to very precisely call the tumors of being lymphoid nature, of epithelial nature or of connective tissue origin and so on and so on.
And by means of this, nowadays, there was a transition within this specialty of pathology for more autopsy-based research towards highly modern diagnostic specialty dealing with biopsies or resections of diseased organs in order to attempt to call diseases by their names. It's interestingly enough that among the 35,000 diseases that human beings know, about 6,000 can be very precisely diagnosed by means of the very primitive methods we apply to the collected tissues in order to enable microscopic examination.
[Vicky Edkins]
Thank you. And recently, obviously, we've experienced a huge public health crisis across the world, the COVID-19 pandemic. What was your role as a pathologist in understanding COVID and contributing to the COVID-19 response?
[Prof Alexandar Tzankov]
Well, this is like a reflection of the strength of our speciality, namely to deal with emerging or re-emerging diseases. And the autopsy techniques are very contributory towards this. Since by means of autoptic examination of the human body, one can address the effects of a disease, emerging or re-emerging disease on all the human bodies.
It's like one of the very few holistic approaches in modern medicine. And by means of this, it was quite important, and it is still very important in any new emerging disease, to perform autopsies in order to understand what the causative agent of the disease does with the human body. And this was not the case, at least not at the very beginning of the COVID pandemics.
Actually, in March 2020, when the first person died on COVID in Basel, we have been looking at the medical literature for any evidence or for any information about what the virus or the disease does with the human body and actually detected only four case reports of partial autopsies. So there were no systematic studies, in spite of the fact that already 150,000 deaths have been reported. This was alarming since much has been known already about epidemiologics, about some risk factors, about the entryway of the virus to the human body.
But actually, there was no information why people were dying on or with COVID. And the cause of death was always figured out like complicated pneumonia, which was not actually the case.
[Vicky Edkins]
What do you think it is that prevented those autopsies from being carried out earlier? You said there were very few case studies early in the pandemic. What prevented more autopsies being carried out or more pathological research?
[Prof Alexandar Tzankov]
Well, I think the answer is complex. On the one side, there was like politicians or health politicians being scared of what is happening and trying to prevent the infectious chains by just saying, well, people who have died, just don't touch these bodies. You may become infected. We don't want to take the responsibility for perpetuating those chains. Then for several reasons, the autopsies not being paid for or not adequately paid for. In many pathology departments, the facilities to perform those autopsies are kept under-equipped or not equipped for highly infectious diseases.
In addition, there are some, well, false attitudes within the society of pathology, but also within medical doctors and within the society, what to expect from an autopsy and what not to expect from an autopsy. In many aspects, modern medical doctors think that by means of applying diagnostics, molecular diagnostics, imaging diagnostics to the living that they almost know everything about the patient before he died and that the added value of performing an autopsy is quite low. But obviously, this was not the case as it was not the case also in other, smaller pandemics, for example, the Zika virus or many years ago along the first years of AIDS and HIV infections and so on and so on.
And at least from our perspective and from what happened here, it was quite important to understand why do people die on or with COVID? And this was not clear at the time we had been facing the first dead persons in Basel.
[Vicky Edkins]
What do you think it is that shifted those perceptions and enabled research to start being carried out? Is there kind of one moment at which your research became possible or was it a kind of accumulation of different effects?
[Prof Alexandar Tzankov]
Well, I think it's a little bit a special situation in Basel, the special communication with our clinicians and also the still high value of autopsies, at least in our own perspective as diagnostic pathologists that we preserved over the years. There's a kind of a tradition in Basel to try to perform as much as possible autopsies, to be quick with reporting those data to and with the clinicians and discussing and trying to learn as much as possible out of those things. And based on that, despite all over, the autopsy rates are falling.
For the last five years, there was like constant autopsy rate in Basel of approximately 15 to 20% of all patients who have died within the system of the University Hospital. And we tried to keep this rate alive with a lot of efforts. And because of that, I think our clinical colleagues were quite persuaded and quite confident that they will gain additional knowledge by ordering these autopsies, discussing with the relatives to give an informed consent for those autopsies to be performed.
And actually, this was successful. I can recall when the first autopsy invitation came, I discussed the issue with the former boss of our department and we put together our potential disadvantages and advantages of doing that and decided to be very precautious, of course, but to perform those autopsies. And we also decided to keep the potential infectiousness or infectious chains as low as possible by assigning the responsibility of performing these autopsies to just two persons.
Actually, me and a colleague of mine performed all the autopsies on these days. And we even did not ask our specific technical preparator to assist us in order to prevent an infection. But using precautious devices that we have been used to to apply for other infectious diseases and using a specially equipped part of our autopsy theatre, I was quite confident that the risk for us as exposed persons would be quite low.
And indeed, after performing the first 10 autopsies, we saw some recurrent and repetitive changes within the human body that helped us to better understand what is going on and why patients die in spite of being, for example, respirated. And also, we understood that the main focus or the main cause of that is not a simple pneumonia, but just a more complicated disease.
[Vicky Edkins]
You received funds as part of BRCCH's fast-track COVID funding scheme to carry out research into the effects that COVID had on the heart of people who had died from COVID. Can you tell us a little bit about the background to that research and why it was needed?
[Prof Alexandar Tzankov]
After the first 10 and after the first 20 altogether autopsies, we realised that COVID is a multifaceted disease, kind of an angiocentric syndrome with the propensity of the human body to develop thrombosis and especially micro-thrombosis. We saw that in spite of the lung being the first target organ, there are changes throughout the human body, either induced by the virus or by the inflammation. And we were convinced that examining every single organ system out of the tissues that we collected at those autopsies would be quite important to gain knowledge for the people, for the living people suffering from acute COVID.
And at that moment, there was this BRCCH call and we realised that with the generous support of a foundation, we would be much more successful in performing all this research and applied for it and were quite happy to get funded. By means of this, we established a COVID research group in our department and I think did the best possible out of what we have collected along especially the first 30, 40 autopsies.
This is reflected by a huge body of papers. I think nowadays there are over 60 and those papers have not only been published, but published in good journals. But most importantly, they have been nowadays cited over 20,000 times. So obviously, what we discovered impacted other people's research, but also some of those aspects found even their way into clinical medicine. So back from the autopsy theatre to the beds of the living.
[Vicky Edkins]
So what implications did the understanding that you developed from that research have for people who might be more susceptible to COVID, for example, and how it should be treated, diagnosed?
[Prof Alexandar Tzankov]
Absolutely. So I think the most important uncovery was this propensity for developing thrombosis of the affected people. This was anticipated by some colleagues already before the autopsies, but the autopsies have proven that this is a major issue in severe COVID.
And because of this and because these findings were then several times confirmed also by other colleagues, thrombosis prophylaxis was then considered part of the treatment of the disease. We were among the first to show the interaction between the virus and the immune system, which paves the way also to significant late complications of COVID, like development of the post-COVID syndrome. Knowledge gained out of the pancreata of the autopsy series explained why COVID worsens the glucose tolerance of the human body at the moment of acute infection, and probably also to a part explained why patients suffering from diabetes are at increased risk for a complicated disease.
Examining the dead persons, we also added an additional risk factor for severe COVID, which was not realized by simple epidemiologic studies, namely the so-called amyloidosis, which is a protein deposition disease. This was not known until then. And there are many other aspects that have been uncovered by means of studying the affected bodies.
[Vicky Edkins]
It sounds like your research is really very important in terms of understanding COVID as it affected the body during that infection period, but also it's been really important in understanding long COVID. Can you say a little bit about how your research is being used still to understand long COVID or post-COVID effects?
[Prof Alexandar Tzankov]
Well, I think it showed the way at least one of the aspects why some complications of long COVID develop, namely the interaction between the virus, the inflammation and the immune answer, which at least to a part paves the way towards the development of some autoimmune phenomenon. So this is one of the aspects. And I think the second aspect here is the microvasculopathy, the thrombotic microvasculopathy that was also uncovered paving the way towards ischemic organ damage, even ischemic organ damage of the brain that also to a part can explain some of the aspects of long COVID.
The practical applications considering long COVID are probably not that impactful as in the acute COVID. Nevertheless, preventing microthrombosis and damping the inflammation along the course of an acute COVID seems to be preventive, at least for some of the feared long COVID effects.
[Vicky Edkins]
Presumably, when you carry out research such as that that you've described, you're not working in isolation in your lab or carrying out an autopsy. Can you talk a little bit about the different people that come together to make this type of research possible and what those different people all contribute to that process?
[Prof Alexandar Tzankov]
Well, actually, in spite of the perception that the pathologist works like a scientific doctor isolated from all the other medical specialties, this does not reflect reality at all. Actually, modern pathologists are incorporated into organ or organ system dedicated teams along the diagnostic radiologist, the oncologist, the surgeons, and many of us actually interact much more with clinical colleagues than in between their cells within the department. A nice example are the breast pathologists who are more or less like a part of a huge team dealing with breast changes and discussing all these things many times a week with their clinical colleagues.
And actually, this also applies to autopsy. The autopsies, at least in Switzerland, are performed with the understanding of the relatives and because of an open clinical question. So actually, the indication to perform an autopsy starts with the clinical question of a clinical colleague who knew the patient before she or he died and who had an open issue by simply not understanding what happened or did the therapies applied before that work, if yes, how, or if not, why, and so on and so on.
So actually, it starts there. And for us, it's very important to communicate with our clinical colleagues before performing autopsy in order to get an idea what the main question is and what happened with the patient before that. Then, after having performed the autopsy, we discuss this autopsy with our clinical colleagues many times on the day of autopsy, and for sure, after having collected and examined all tissues. And finally, we may report this autopsy as a case report or as a case series, performing research out of that.
And actually, all these habits have been also applicable to what happened in COVID and in COVID research. So basically, we interacted a lot with our clinical colleagues from the emergency unit. They have been facing all the problems and complications of acute COVID and had a lot of issues they wanted we to clarify on autopsies. We interacted with our colleagues from the radiology to understand how the radiological image, especially of the lungs, of the affected lungs correlated with what we see on autopsy. Then, after having collected tissues, many basic researchers interested at cellular and subcellular processes were quite interested in getting small pieces of those tissues to examine at the molecular level what changes apply to those tissues, and so on and so on.
And bringing all these things together, we were forced and we wanted to interact with all these colleagues. And along these 60 papers that we have published based on our COVID autopsies, we interacted with almost every medical discipline, maybe with the exception of psychiatry.
[Vicky Edkins]
It sounds like a phenomenal amount of teamwork went into that research. And I think some of the other work that you've done has involved working with an even broader range of people, even historians and genealogists. Is this correct?
[Prof Alexandar Tzankov]
Yeah. This is like a small hobby, not only of mine. But some years ago, we have been asked to look at the bones of a mummified lady who died some centuries ago in Basel, mainly because when the anthropologists performed a CT scan of her body, they uncovered some bone changes and they wanted to know what is hidden behind these bone changes.
And this was the first time in my life to deal with a mummy. But we applied the same techniques we're using for discerning causes of death or causes of disease that we apply to living people or to autopsies of people who recently died of a disease. Actually, we examined those bones, which were quite nicely conserved or preserved, and were able to see changes that are usually seen either in syphilis or in tuberculosis.
And based on that and on the tissues that we collected within this examination, and with the application of modern molecular techniques that have been applied to those small pieces of tissue in Bolzano, actually at the institute that deals with the mummified body of Ötzi, we were able to finally find the causative agent, the causative infectious agent that probably significantly contributed to the death of this person in Basel.
Quite interesting and also, once again, a nice example of an interdisciplinary work, which did not stop only at the borders of medicine, but here a lot of other people, volunteers from the whole canton, from the whole city, spent a lot of work in order to first identify who the lady was. And one of the sensational findings was that this lady was somehow related to Boris Johnson on the one side.
And then also, discerning her cause of death and also understanding why this lady underwent a huge body of medical procedures before dying. Nevertheless, we discovered that the probable causative agent of her lung suffering was a very specific microbacterium, kind of a sister germ of the causative agent of tuberculosis, yet not being the agent of tuberculosis itself. So, quite an interesting finding.
[Vicky Edkins]
It was a really fascinating piece of research. And I think that for me, particularly, it was really interesting how the research that you did kind of came together with the research of lots of other disciplines to kind of really tell a story about that lady’s life and death.
[Prof Alexandar Tzankov]
Absolutely. So actually, this is a nice example how we work and what the role of the pathology nowadays is. We bring a puzzle stone within a huge puzzle of pieces in order to get insight of what a person is suffering from, trying to give to this suffering a name and in many instances, those names are linked to distinct prognoses, to distinct clinical courses, but most importantly to distinct therapies. And, the more specific the therapy is, the more course-related it is, the better the outcomes of the patients.
And, actually, looking at, for example, the cancer statistics of the last 20 years, this approach really seems to work. In many, many cases, cancer has turned nowadays towards a chronic disease, with which people can live with in spite of being a deadly disease. So actually, since 2030 I think, more than half of the patients diagnosed with cancer do not die of cancer. And this turning point of course is due to the efforts of multiple disciplines and one part of those efforts and one contributing stone towards this success is also the development in the pathology, the interaction of the pathologist with other disciplines for the sake of our patients.
[Vicky Edkins]
Thank you. We’ve had a really insightful kind of look at the research that you’ve been doing over the past few years. Turning now to the future, what do you think is on the horizon for you next? Where do you think your research will take you next?
[Prof Alexandar Tzankov]
Well, I’m going back to my specific point of interest. So, I’m performing a lot of research in leukemias and lymphomas and I will back there. My special interest is now in developing new molecular diagnostic tests, especially in patients with relapsing lymphomas so that based on the molecular signature of their first diagnosis and looking for these molecular signatures in the blood at later stages, I can. The idea is if you have a relapse of a lymphoma, usually nowadays the radiologist has to go with a needle, obtain tissue and send it to us in order us to say is it a relapse or not. I can look for the molecular signature of the lymphoma in the blood and by means of this, I can offer an alternative way of approaching those patients and preventing them from being exposed towards a very invasive medical procedure for the diagnostics. The method is called liquid biopsy. It’s already used in other types of cancer, but not used hands-on in lymphomas and I would like to set up a valid medical procedure for diagnostics of lymphoma relapses in that consideration.
[Vicky Edkins]
Professor Tzankov, thank you ever so much for taking the time to talk with me about the research that you did in COVID, about your research with the lady in the church and also what awaits you in the future. It’s been an absolute pleasure talking with you so thank you very much.
[Prof Alexandar Tzankov]
Thank you very much once again.
[Vicky Edkins]
Thank you for listening to the Science Speaks: Conversations on Health podcast. This podcast was produced by the Basel Research Center for Child Health in Switzerland and hosted by me, Vicky Edkins. Editing was carried out by Sebastian Schell at the University of Basel's New Media Centre. Thank you to our generous funder, Fondation Botnar, and our partner institutions, ETH Zurich and the University of Basel, for your ongoing support.
If you would like to learn more about what's happening at the BRCCH, you can visit our website at brc.ch for information on our upcoming events and to sign up for our newsletter. We're also on social media. Follow us on LinkedIn at the Basel Research Center for Child Health or on X, formerly Twitter, @BRC_CH.

Operating theatre at the University Hospital Basel
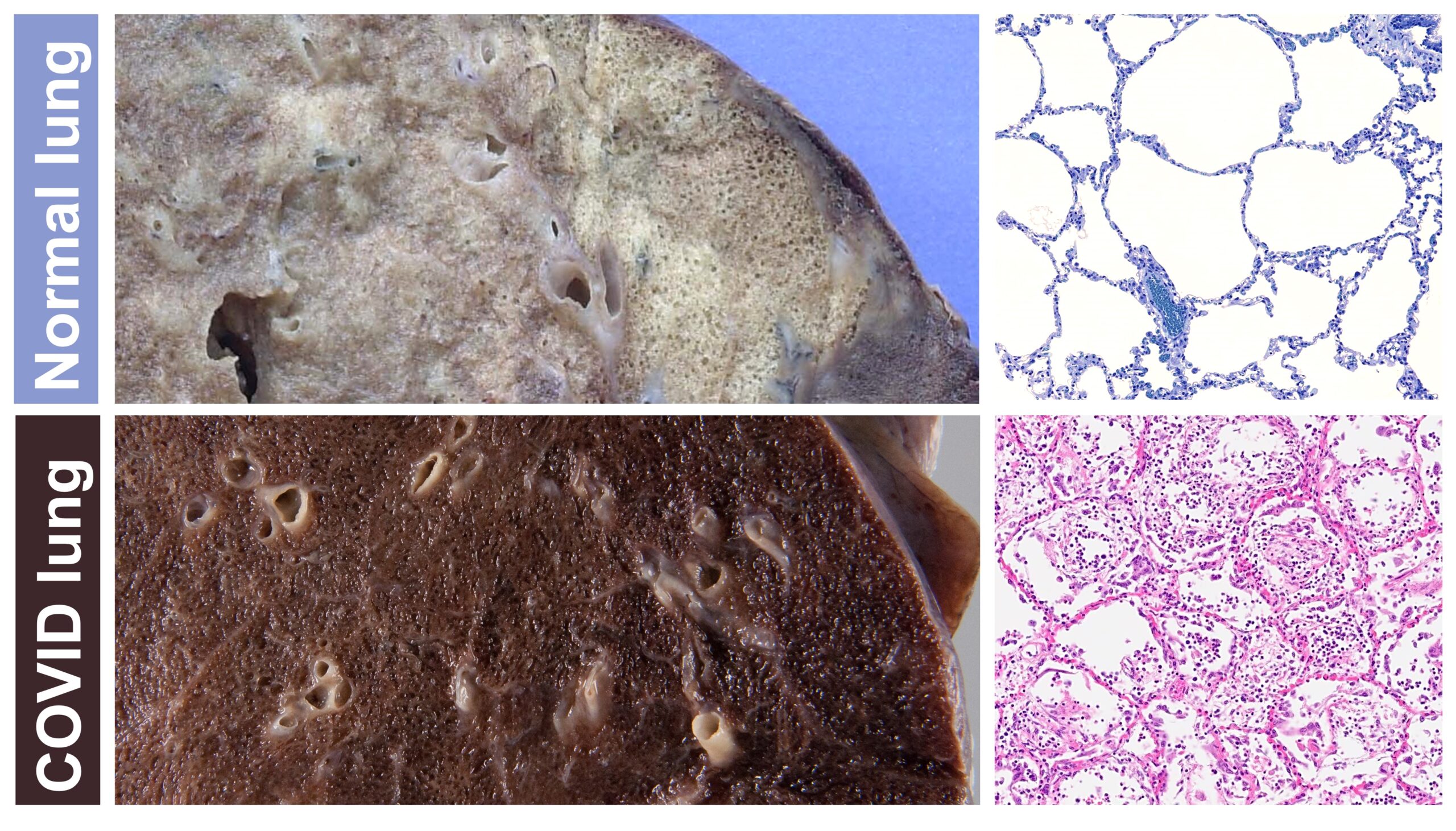
Comparing the appearance of a lung in a patient who died from COVID-19 to that of a patient without COVID
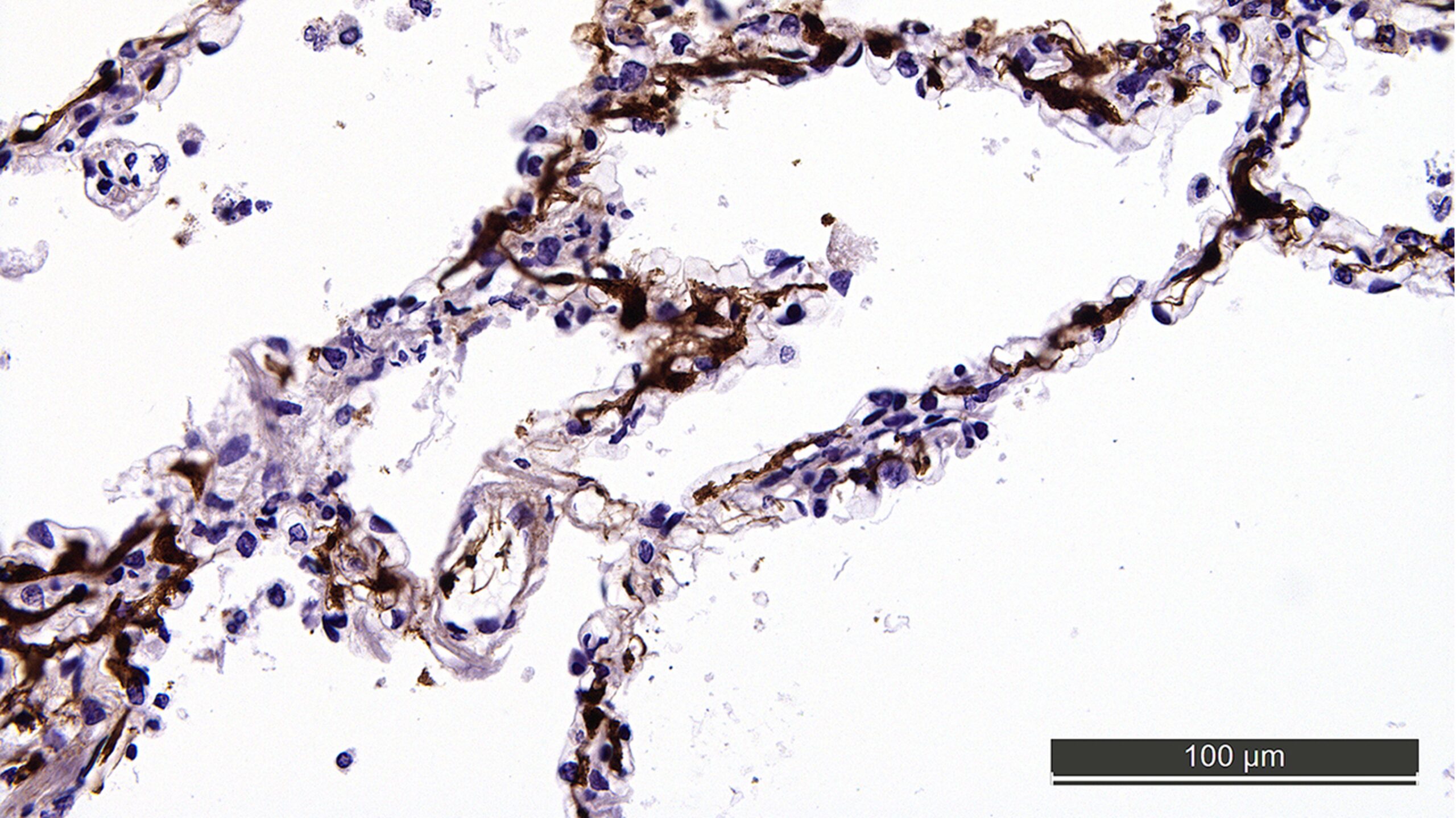
Microthrombi (brown stain) obstructing the microvessels of the lung in lethal COVID-19
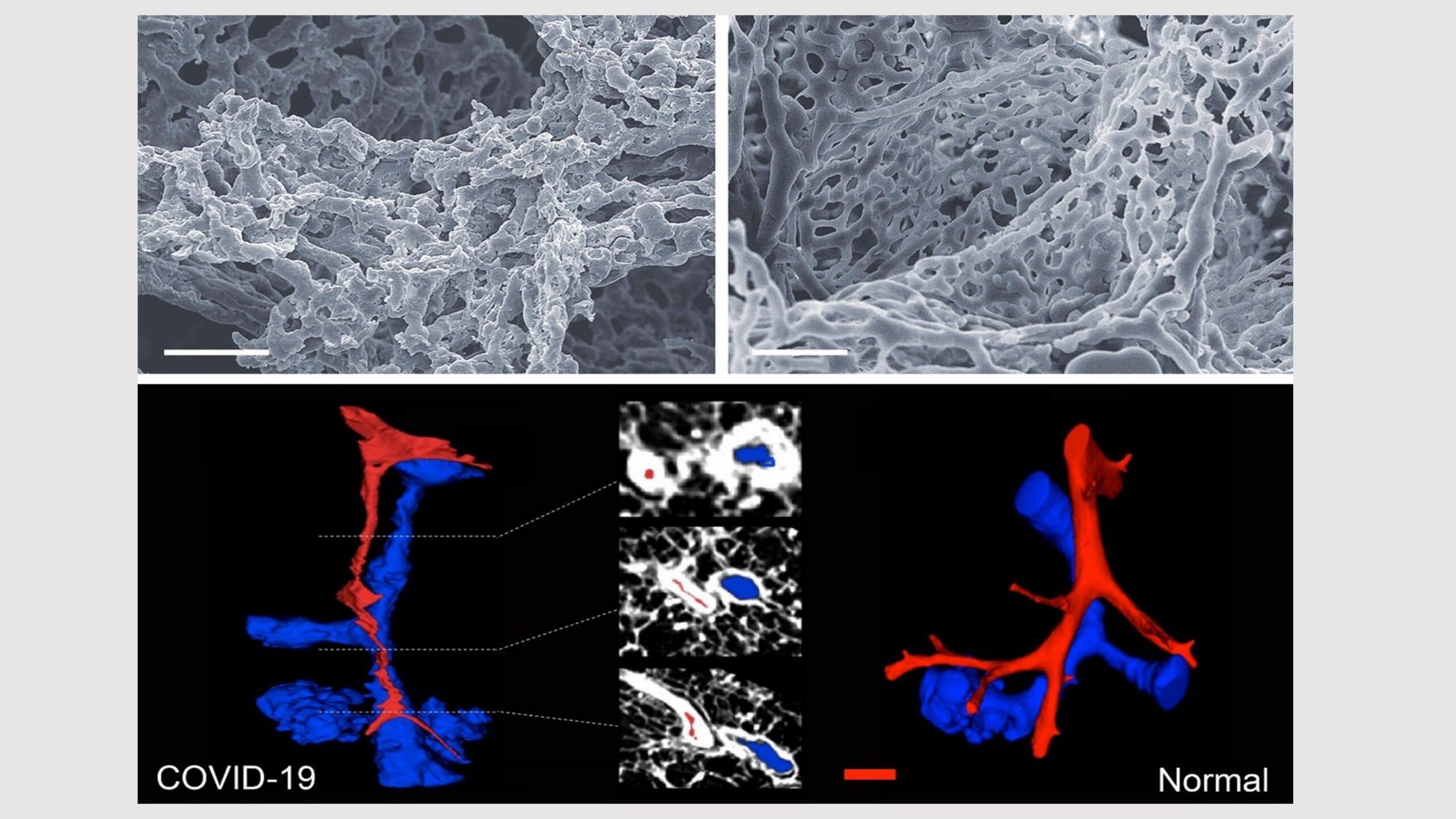
Microvascular perturbation of the alveoli in COVID-19
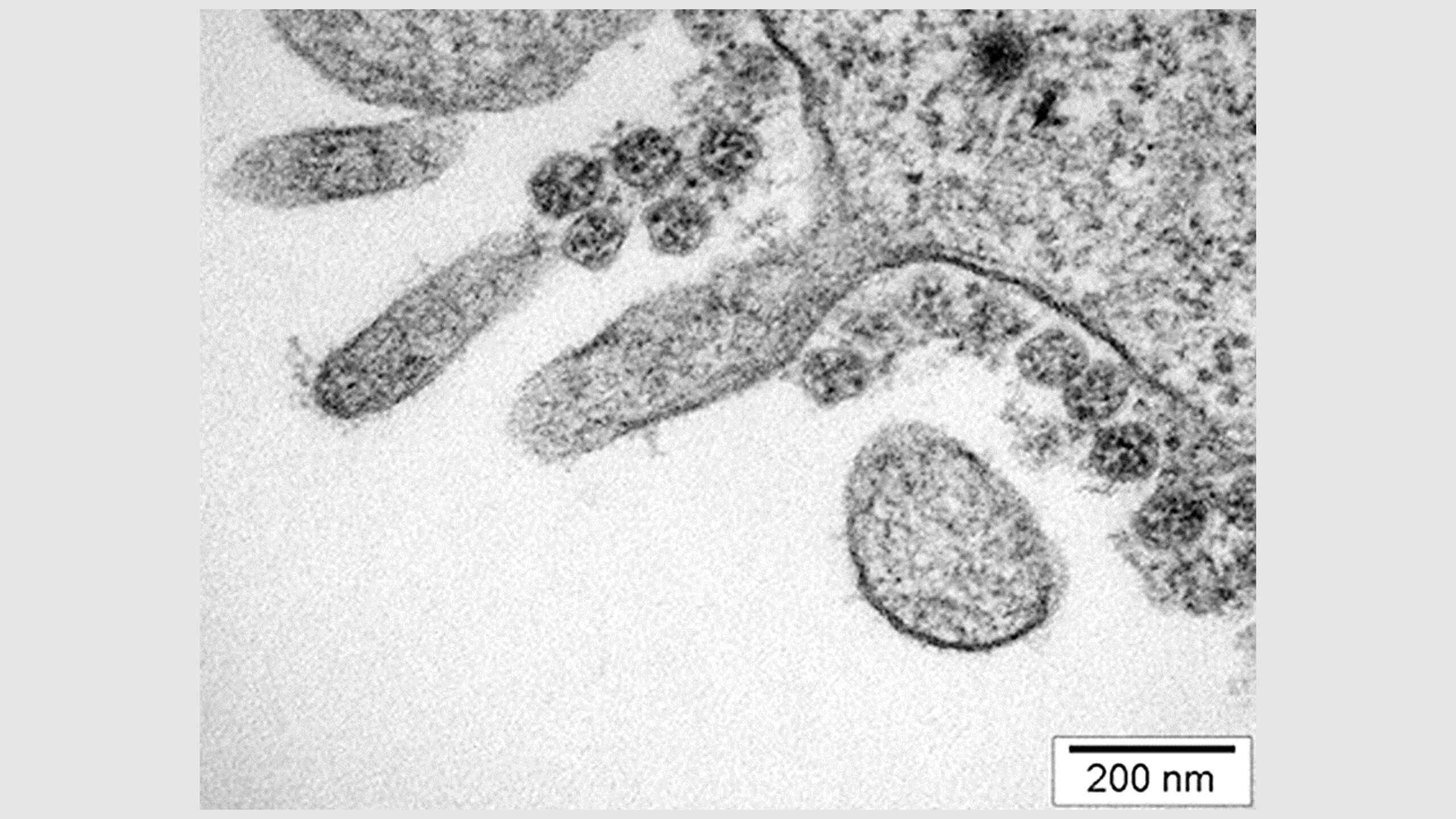
SARS-CoV-2 on the surface of a villous epithelial cell